ABOUT EVENT
Welcome to the EHS for Diagnostics & Medical Devices Summit
Industry’s Definitive Safety Initiative Dedicated to Achieving Operational Excellence Within the Highly Regulated Medical Device Sector
The EHS for Diagnostics & Medical Devices Summit united 70+ EHS VPs, Directors & Site-level heads from the world's leading diagnostic and medical device companies for 3 days of forward-thinking discussions, case study presentations, interactive workshops, networking sessions and more.
Your EHS Peers United to:

ENCOURAGE
Encourage new initiatives to standardize EHS processes across multiple sites globally and sustain wellbeing and culture across R&D, manufacturing, and distributive workforces. Implement a best-in-class EHS program and align with ISO and OSHA standards to avoid expensive compliance violations.

HEAR
Hear the latest innovations to achieve zero pollution and foster environmental sustainability. Effectively navigate diverse environmental guidelines regarding material usage and medical device waste management to promote a more sustainable workplace.

SHARE
Share best practices on ergonomic risk mitigation, electrical and chemical safety, and reducing additive manufacturing hazards associated with occupational exposure to dust, fire, and explosion risks. Promote cross-functional collaboration to factor safety into product and manufacturing process design.
Gain Points Towards Your Recertification
 |
By attending this safety initiative, you could earn points towards your recertification, meaning you can earn while you learn. |  |
BGC® Diplomates can earn up to 15 technical contact hours (or 2.5 CM points) for this event. See http://www.GoBGC.org/practitioners_CIH_educationalevents for more information.
BCSP Diplomates can earn up to 15 technical contact hours (1.5 points) for this event
This event has also been approved by ABSA International for 0.125 CM points toward RBP/CBSP recertification



Who Was in Attendance?
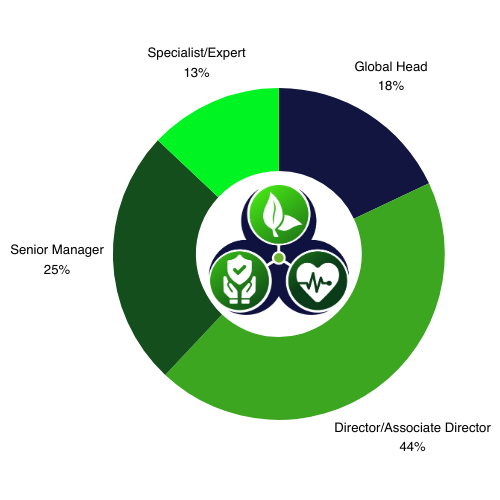
*Numbers based on other events in the EHS series
